Advances in the response of surface-subsurface water exchange to activities of typical aquatic organisms in the hyporheic zone of rivers
-
摘要: 河流潜流带是地表-地下水连通和交换的主要区域, 地表-地下水过程不仅促进了生源物质的迁移转化过程, 还能涵养水源、稳定区域生境, 为水生生物提供良好的栖息环境。因此掌握水生生物活动与地表-地下水交换关系是深刻认知和科学保护水生生态系统的关键。本文综述了前人有关水生生物活动反馈于地表-地下水交换过程的研究, 例如, 底栖微生物形成的生物膜可以吸收或滞留生源物质, 改变迁移的时间和路径; 水生动物的行为可能通过改变河床渗透系数和孔隙率等物理参数影响各类物质的地表-地下水交换通量; 水生植物对水流的阻滞和扰动也会作用于地表-地下水交换过程。基于目前研究, 本文提出了该领域的3个未来研究方向: 潜流交换和水生生物互馈理论, 水生生态功能与地表-地下水相互作用关系, 河流潜流带生物-地球-化学耦合过程。Abstract: The river hyporheic zone is the main area of surface-subsurface connection and exchange. Surface-subsurface water exchange promotes the transport and transformation of biogenic substances and can conserve water resources, stabilize regional ecological environment, which provide a great habitat for aquatic life. Therefore, understanding the relationship between the activities of aquatic animals and surface-subsurface water exchange is the key to deep cognition and scientific protection of aquatic ecosystems. This paper reviews previous studies on the feedback of aquatic activities to the surface-subsurface water exchange process, for example, the biofilm formed by benthic microorganisms can absorb or retain biogenic substances, prolonging and changing the migration time and path of substances; the activities of aquatic animals can affect the physical characteristics of riverbed, such as permeability and porosity of streambed, which in turn affect the hyporheic exchange flux and substances transformation efficiency; the block and disturbance of water flow by aquatic plants can also affect the surface-subsurface water exchange process. According to the current researches in this field, three important research directions of the relationship between river hyporheic exchange and aquatic organisms are proposed in this study: the theory of mutual feeding between hyporheic exchange and aquatic organisms; relationship between surface-subsurface water exchange and aquatic ecological function; biogeochemical coupling processes in hyporheic zone.
-
随着河流水环境、水生态相关研究的深入,河水和地下水交混区域——潜流带的生态学研究成为一个热门话题。潜流带是位于河流底部与河床顶层,并延伸至河岸两侧的水分饱和沉积物层[1]。作为地表水与地下水联系的纽带,潜流带不仅是氮、碳等物质循环的重要场所[2-5],也是河流生态系统重要的一环,为水生生物提供栖息、觅食、产卵等场所[6-8]。例如,每年全球陆生有机碳总量中约25%的运输、转化、储存量在河流中完成,约65%的反硝化反应发生在沉积层中[9];河流水动力驱动下的地表-地下水交换,能驱动各类物质在潜流带中的滞留、迁移与转化,此过程也称之为潜流交换。根据水动力作用原理,潜流交换主要有2种: 对流交换(又称为泵吸交换)和冲淤交换[9]。在潜流带发生的物质交换主要包括保守性溶质[4,10-12]、反应性溶质[13]和颗粒物等[14]交换过程。
河流生态系统的物理和生物属性以一种复杂的方式相互作用[15],研究水生生物活动对地表-地下水交换的作用是对水生生态系统平衡进行深刻认知的关键环节。人们对河流潜流带最早的认知可以追溯到20世纪30年代对潜流带生物的认识[16]。1974年发表在Sinence期刊上的一篇论文研究了潜流带与水生生物的关系,并指出河流潜流带为生物提供了良好的栖息场所[17]。之后的研究发现,水生生物自身的活动也会影响河流的潜流交换过程[18],进而改变河流生态系统的能量流动与物质循环等生态功能[19]。多年来,国内外学者在河流潜流交换对水生生物活动响应的方面开展了大量研究,如潜流带底栖微生物形成的生物膜可以控制区域潜流交换通量[20];水生动物进食、排泄、建造虫洞等生命活动,可以显著改变物质潜流交换时间及深度[21];水生植物的存在会引起局部压力梯度,进而对潜流交换通量的改变产生重要作用[22]。因此,系统理解潜流交换过程对水生生物活动的响应机理具有重要意义,然而已发表的相关成果只关注到部分水生生物对潜流交换的影响,缺少包含微生物、动物及植物等全面且系统的综述。
为了更全面地理解河流潜流交换过程对水生生物活动的响应机理,本文选取典型水生生物,包括底栖微生物、水生动物和水生植物3大类生物,探讨它们的主要生命活动对潜流交换的影响: 底栖微生物形成的生物膜附着以及自身结构对潜流交换过程的影响, 水生动物中的大型底栖无脊椎动物和鱼类活动对潜流交换的影响, 水生植物的存在通过直接和间接的方式改变潜流交换通量。综上,本文提出了3类未来研究展望: 潜流交换和水生生物互馈理论, 潜流交换与水生生态功能关系,潜流带生物-地球-化学耦合过程。
1 底栖生物膜对地表-地下水交换的影响机制
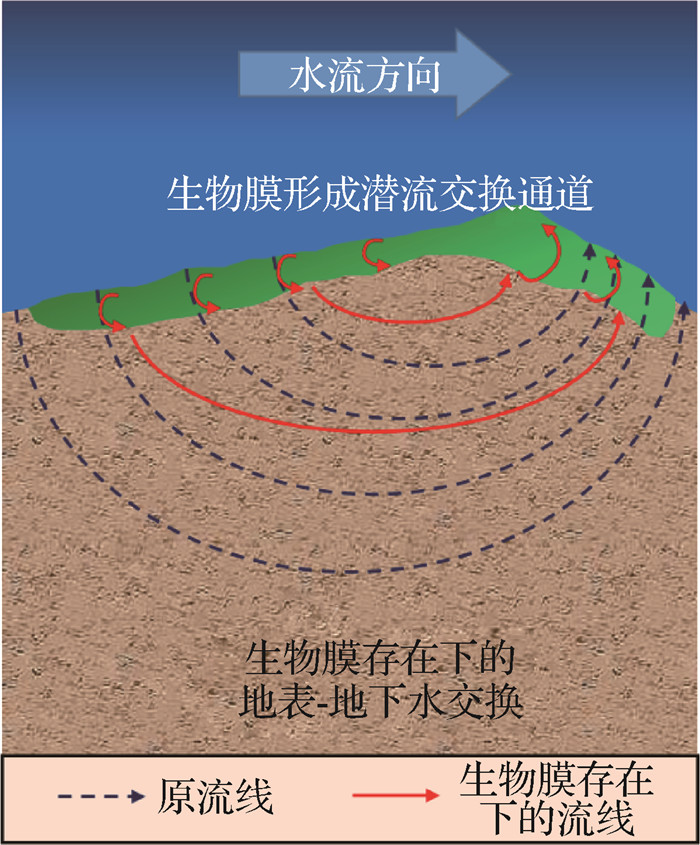
潜流带中的微生物主要包括藻类、细菌、真菌和原生动物,微生物的生长会分泌胞外多糖,形成能够覆盖于河床的生物膜[23-24]。潜流带中的底栖生物膜结构对无机物和有机物的转化效率较高,能有效促进物质循环,并对污染物起到较强的降解作用[25]。底栖生物膜的附着及自身结构都可以改变潜流交换通量,影响各类物质(尤其是生源物质)的地表-地下水交换过程(图 1)[26]。
1.1 生物膜附着对地表-地下水交换的影响
生物膜的附着能够使河床沉积物保持稳定,形成区域生物堵塞,进而控制区域生化反应过程,影响生源物质的潜流交换通量[27-28]。潜流带中的岩石、鹅卵石、泥沙、生物体残骸以及底栖植物叶片等为底栖微生物提供了大量的可附着表面,从而形成底栖生物膜共生的环境,其中细菌、古菌、藻类、真菌、原生动物和病毒都是生物膜基质的重要组成部分[29-30]。底栖生物膜对潜流带水动力结构和生源物质梯度的形成起到至关重要的作用,其复杂的多孔系统结构能够有效保护生物有机体免受水流冲击[26]。
生物膜附着于细颗粒沉积物上,可以形成黏性基质,将沉积物颗粒紧密包围并嵌入其中,对稳定沉积物、抵抗再悬浮具有重要作用[27]。此外,生物膜的附着还会引起河床的局部区域物理性质发生改变,从而降低沉积物的渗透性,减缓或阻止某些物质进出潜流带的过程[28,31-33]。
生物膜引起的生物堵塞控制着区域生化反应过程,影响了生源物质的潜流交换通量和生物地球化学转化速率,对潜流带的物理和生物地球化学过程具有较强的调节作用。Ping等[28]建立了水流-溶质-微生物代谢耦合过程模型,用于描述生物膜的堵塞作用机制。Caruso等[34]发现细菌可通过附着于生物膜的形式介导沉积营养物质的转化,而微生物生长和生物膜引起的生物阻塞可以限制潜流交换的速率和模式,这种效应被称为减慢生化反应[18,34-35]。
1.2 生物膜自身结构对地表-地下水交换的影响
底栖生物膜作为复杂的生物群落,是物质流入或流出潜流带的必经通道,其复杂多变的结构可以增加物质传输时间和传输距离,进而控制潜流交换通量[26]。生物膜与沉积物之间的相互作用可以改变多孔介质的物理结构,影响多孔空间中生物膜的生态环境。跨细胞与沉积物尺度的生物膜结构和功能之间的耦合则有助于其作为微生物的“皮肤”得以表征,以滞留、放大、转化反应性物质和微生物本身,从而控制物质的潜流交换通量[31-32]。
底栖生物膜表面的物理结构特征会对胞内外物质输运过程产生影响,改变一些物质的潜流交换通量。为了提高营养物质的摄取,底栖生物膜会形成树冠状和指状结构来增加生物膜的表面积,以补充关键溶质的摄取,在上覆水养分浓度低的情况下,这种结构更易加剧营养物质向膜内迁移[36];为了应对高水流流速所导致的表面高剪应力,底栖生物膜会形成几厘米长的顺水流方向的脊线[37],改变上覆水流态,形成局部涡旋结构,从而影响区域潜流交换通量[38]。
底栖生物膜还会通过其内部的通道系统影响潜流交换。Battin等[39]发现潜流带中的生物膜含有空洞和通道系统,其中的通道系统充当了营养物质的传输路径。由于地表-地下水交换作用,泥沙、污染物甚至是毒性物质会被带入底栖生物膜内,并在其中传输[40],从而影响生物膜内部的生态结构。缓慢的水流可以使通道系统在生物膜内迅速生长,而在流速较大的水流中,生物膜的生长分布往往更加紧凑[37]。因此,这种生物膜相互耦合的物理结构有利于膜内生源物质分布和生物新陈代谢[41]。
2 地表-地下水交换对水生动物活动的响应过程
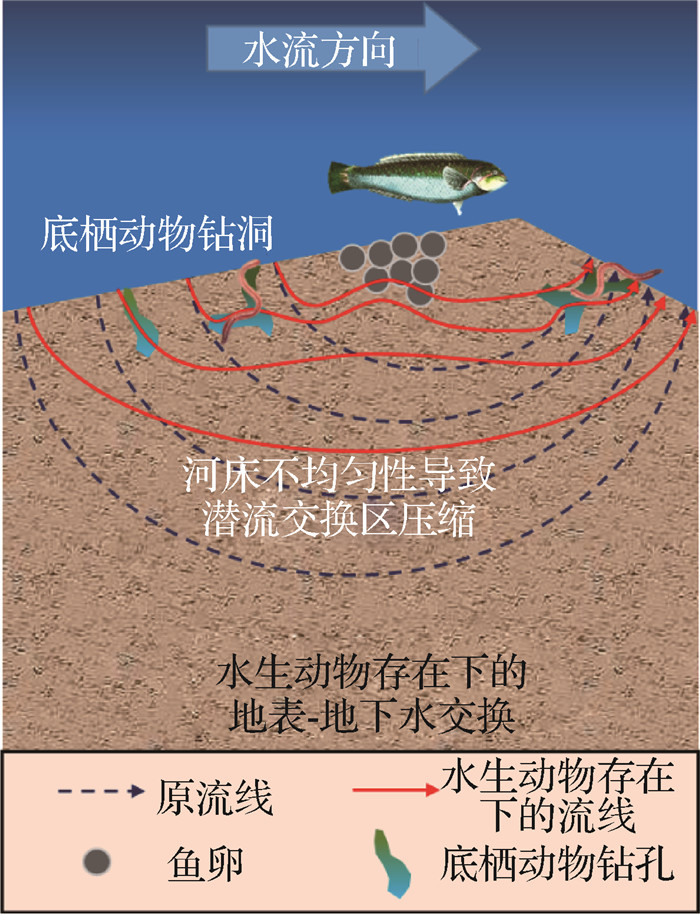
生活在潜流带中的水生动物主要包括节肢动物、软体动物等大型底栖无脊椎动物以及鱼类,其生物扰动作用对于潜流带内物质迁移过程和转化效率的影响尤为重要[42-43]。大型底栖无脊椎动物进食、排泄、运动和建造虫洞等生命活动,可以显著改变潜流交换中物质的滞留时间和迁移深度[19];鱼类的产卵等活动通过改变河床地形诱导泵吸交换,并可通过筛除细泥沙和疏松河床,从而改变河床渗透系数和孔隙度[44]。由此造成的潜流交换过程的改变对于河流生态系统的整体功能有着直接影响(图 2)。
2.1 大型底栖无脊椎动物活动的影响
由于潜流交换具有调节水动力和生物化学过程的作用,潜流带成为了一些大型底栖无脊椎动物的优良栖息地[23,45]。潜流带中的大型底栖无脊椎动物主要摄取底泥中沉积的有机物碎屑,以及微生物与藻类共同形成的底栖生物膜[46];同时,大型底栖无脊椎动物也为一些鱼类、水鸟等提供了食物来源[47-48]。大型底栖无脊椎动物觅食和栖息等行为会对河床表面及其内部物理性质造成影响,从而改变地表-地下水交换过程[46],尤其是河床中迁移活跃的腹足类和端足类等大型无脊椎动物,其生物扰动作用更为明显[23]。因此,大型底栖无脊椎动物对于潜流带生态系统的物质循环和能量流动过程起到至关重要的作用,是维持河流生态平衡的重要类群[49]。
大型底栖无脊椎动物在潜流带中栖息会影响河床的表面性质。由于大型底栖无脊椎动物的栖息,河床表面流速会发生改变,导致细颗粒泥沙发生沉积或再悬浮,进而潜在影响潜流交换通量和生物化学物质分布[50]。Macdonald等[51]发现大型底栖无脊椎动物可以通过建立丝网来改变河床的表面性质,从而限制潜流交换,并使得潜流带各类物质的滞留时间发生改变,进而增加潜流带内单位体积生物代谢对河流溶质的需求。
潜流带中的大型底栖无脊椎动物可以通过取食和钻洞等活动影响河床内部物理条件,进而影响河床内部的潜流交换过程[52]。Nogaro等[53]发现大型底栖无脊椎动物的生物扰动作用会使得潜流带的孔隙度得以保持,从而确保其栖息区可存在适宜的营养物质和溶解氧通量。大型底栖无脊椎动物在潜流带中的扰动行为,加剧了河床渗透系数的不均匀性,延长了溶解氧或营养物质的潜流交换路径和时间,使得河床深处形成贫氧区或营养贫乏区。此外,其生物扰动会增加河床的不均匀性,在河床上部形成优势通道,进而压缩潜流交换区域[54]。
2.2 鱼类活动的影响
如今,由于自然环境的变迁和人类活动的影响,一些鱼类无法适应新的生存环境,种群数量正在急剧减少[55]。潜流带作为鱼类的筑巢地和产卵场,其生境将直接影响鱼类的生存和繁衍,其中潜流交换过程是保持潜流带生境生化条件稳定的一个重要因素[56]。此外,鱼类的生命活动也会对潜流带生境产生影响,总体上,鱼类的产卵和觅食主要通过对河床地貌产生影响,从而改变地表-地下水交换过程,并进一步影响至潜流带生态环境。因此,研究鱼类和潜流交换之间的关系对于鱼类乃至整个河流生态系统的健康都尤为重要。
鱼类对潜流交换的影响主要表现在2个方面: 一是其产卵行为会影响到潜流交换和溶解营养物质的运移;二是其觅食行为会影响河床的地形结构特征。
首先,鱼类的产卵行为会影响到潜流交换和溶解的营养物质的运移[44]。Montgomery等[57]通过实体测量,证实了鲑鱼产卵引起的河床地形改变,会诱导泵吸交换、筛除细泥沙和疏松河床,从而改变河床渗透系数和孔隙度。此外,无论是高密度还是低密度的鱼类产卵行为,都会导致河床沉积物的混合,通过清除更细的颗粒、粗化和重整河床表面颗粒,使得颗粒间的填充物更加松散[57],从而导致河床形态的改变。松散的介质促进了地表-地下水交换过程,为鱼卵带来了更多的溶解氧,同时也清除了卵坑中由于鱼卵新陈代谢所产生的废物[58],有利于鱼卵的孵化。
其次,底栖鱼类的觅食行为不仅可以改变细泥沙淤积的性质和速率,以及粗粒河底的结构和地形,还可能对河床的物质特征、颗粒卷吸阈值(Particle entrainment thresholds)和河床的输沙通量[59]造成影响。如幼年马鲅鱼的觅食行为会改变河床表面的砾石、破坏结构的稳定、增加微地形粗糙度[60],并导致细砾石层临界剪切应力减小。此外,Statzner等[61]研究表明,多种鱼类对沉积物迁移率的共同影响小于各单类物种的累加影响。
3 地表-地下水交换对水生植物的响应机制
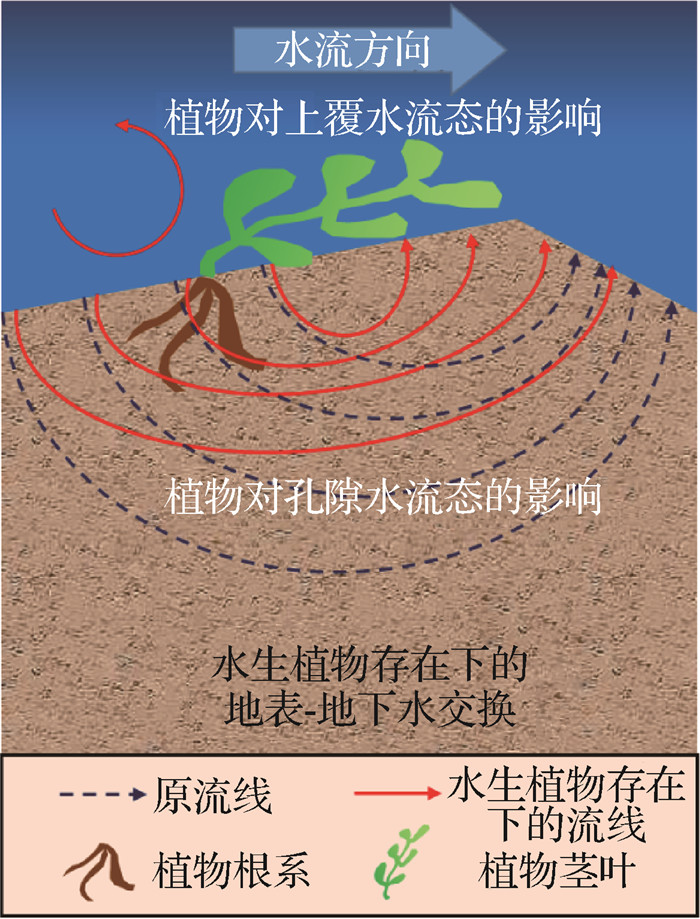
在潜流带中,植物的分布会对区域的水动力条件、养分分布和沉积物分布产生一定影响[20,62-63], 水生植物主要通过对水流流态以及底栖生物生活环境的改变,直接或间接地影响潜流带生态环境以及地表-地下水交换过程(图 3)[20]。水生植物在河流中的生长会作用于地表水[64-65],进而直接改变潜流交换通量;底栖水生植物可以改变河床物理特性,为底栖生物营造合适的生境,进而间接影响潜流交换。
3.1 水生植物的直接影响
河流中常见的水生植物种类有芦苇、香蒲等,根植于潜流带的水生植物会影响上覆水和孔隙水的流态,从而影响水和其他物质的潜流交换通量,最终对潜流交换产生直接影响[62-63,66-67]。
水生植物通过改变水流流态对潜流带生态环境造成潜在影响。水生植物能够改变上覆水的平均速度和湍流结构,从而影响沉积物和污染物的输送[68]。潜流带中的水生植物会增强湍流的紊动强度,甚至引起涡流,这些涡流的存在对泥沙沉积、水生植物种子的散布和上覆水体中的生物化学反应具有重要的影响[64]。Siniscalchi等[69]通过对照试验发现,水生植物存在时,上覆水流态改变明显,湍流动能显著增加,水流涡旋结构更加复杂。水生植物覆盖河床会对水流施加阻力,降低水流在其内部和横向的流速,从而使无机物和有机物细颗粒稳定沉降聚集于河口潜流带区域[38,65]。
水生植物还会阻滞和吸收进入河流中的碳、氮元素和其他有机营养物[70]。若河流某区域集中分布有大型植物,则流经该区域的物质会发生短时间的被储存和滞留效应,其迁移过程也会被相应延缓[71];且河岸带湿生植物的密度和空间分布结构会影响洪水期的水面波动[72],从而影响潜流交换通量。此外,Curran等[38]发现生长于河口、河岸带的大型植物与此区域的水生植物共同成为天然的拦沙屏障,加速泥沙在该区域的沉积,从而改变一些物质的地表-地下水交换过程。
3.2 水生植物的间接影响
水生植物不仅会对潜流交换造成直接影响,还可以通过改变河床物理特性和改造底栖生境的方式来间接影响潜流交换[67]。水生植物扎根于潜流带,改变了河床性质和地下水流态,使潜流交换通量发生变化;水生植物为底栖微生物和大型底栖无脊椎动物营造了一定的生境,并通过它们的生命活动间接影响潜流交换(图 3) [4,73-75]。
从水生植物对河床产生影响的角度来看,其作用是双向的。一方面,底栖植物可通过增强河床的固结和附着力来降低河床沉积物的流动性[76],从而对潜流交换速率产生一定的负面影响; 另一方面,White等[77]发现水生植物也能引起河床渗透性增加,这是由于水生植物的根系通过季节性的生长和萎缩,导致沉积物增厚以及大孔隙的产生。水生植物还能影响河口泥沙沉积特性,改变河口潜流带的地形条件,影响水和其他营养物质甚至包括污染物的潜流交换通量,进而改变河口潜流带生境[66]。
水生植物的存在为底栖微生物和大型底栖无脊椎动物营造了合适的水动力条件[74]。由于水生微生物、大型底栖无脊椎动物的生长繁殖与水流速度及湍流呈负相关关系,水流流态会严重干扰微生物和大型底栖无脊椎动物的取食和交配行为,进而使得它们通过其物理运动、产气以及由于产生分泌物和产卵造成的沉积物增厚等方式,对地表-地下水交换产生影响[67]。水生植物的茎叶以及根系系统能为微生物和大型底栖无脊椎动物提供良好的栖息环境,进而促进生源物质吸收和转化,提高水体的自净能力[74]。
4 不同水生生物对地表-地下水交换的联合作用机制
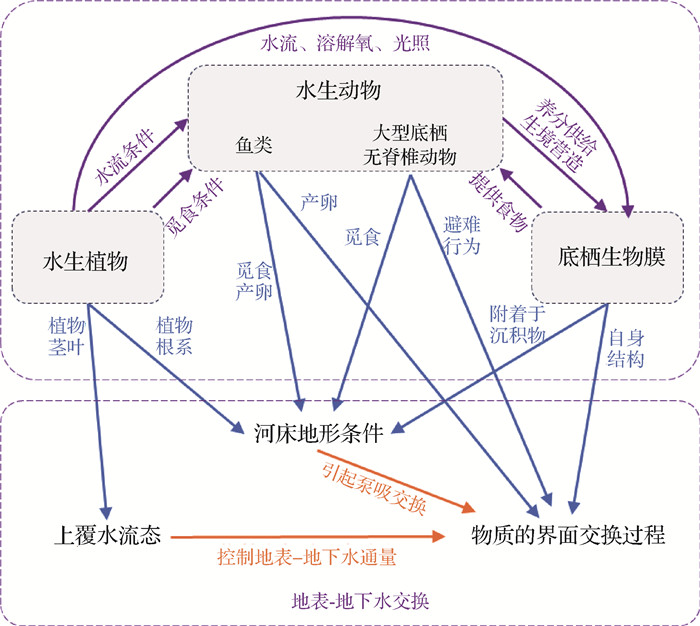
水生动物与水生植物都与沉积物-水界面之间存在着一定的物理、化学及生物联系,其间的相互作用可能会对生物多样性产生正面或负面的影响,改变潜流带的理化性质,进而影响生源物质的潜流交换过程,因而研究多种生物之间的相互作用及其对河流潜流带的联合作用机制至关重要(图 4) [4,16,78]。例如,水生植物根系为底栖生物提供新的栖息地,增加河流沉积物孔隙度,从而促进氧气进入沉积物,刺激底栖微生物和大型无脊椎动物的活动,进而改变潜流带水动力特征[78]。
4.1 水生植物主导的联合作用机制
研究表明,河流中一些水生植物的存在可能通过影响其他水生生物活动来改变区域潜流交换过程[79-80]。水生植物可以控制阳光进入水体的通量、河流的水文过程、食物资源分布以及生物化学环境[78,81],为潜流带底栖微生物和大型底栖无脊椎动物提供合适的生境,增强生物群落多样性[82],进而与其他生物活动联合作用于潜流交换过程[74]。下文分述水生植物茎叶和根系与其他水生动物对潜流交换过程的联合影响。
水生植物茎叶可为其他水生生物提供合适的生境,茎叶的存在与生活在该区域的生物会联合作用于潜流交换。从整体来看,水生植物茎叶会引起水流速度和湍流强度等水流流态变化,严重干扰底栖微生物和大型底栖无脊椎动物的取食和交配行为,与其生长繁殖呈负相关关系,如流速的减小使得底栖微生物和大型底栖无脊椎动物生命活动更加活跃,其分泌物、产卵和粪便将导致沉积物增厚,减小潜流交换通量[67]。从局部来看,水生植物的物理结构会增强局部流态的复杂性,提高区域泥沙沉降速率,减少养分在沉积物-水界面的通量,从而在潜流带中营造复杂的含氧条件[83]。一方面,这些影响导致了潜流带无脊椎动物,特别是穴居动物的多样性减少[84];另一方面,复杂的氧气条件增加了微生物的多样性,如硝化菌和反硝化菌或硫酸盐还原菌和硫化物氧化菌等[85],这些生物群落变化又会反作用于潜流交换通量。此外,大型植物的存在可以阻碍捕食者在河床附近的移动和觅食,减少对潜流带内栖息生物的捕食行为[86],使部分底栖生物的丰度得以保持,并影响潜流交换。
根植于河流潜流带中的植物根系也会直接影响水生生物群落,并联合作用于潜流交换过程。从整体来看,植物的茎和根增加了水生环境中沉积物的稳定性[78],这种相对固定的潜流区域有利于生物群落的多样化[87-88]。Walker等[89]通过对野外所采试样进行多元化分析得出,微生物和无脊椎动物的滞留能力在相对稳定的沉积物中会得到增强,从而增加其物种数量或丰富度。就局部而言,沉水植物的存在会引起地表-地下水界面的区域上升流和下降流,由于潜流带中的水生生物群落对水动力条件的变化极为敏感,随着水位、流量、洪水频率和地下水水质的变化,其物种组成和丰富度会随之发生改变[90-92]。植物根系所形成的复杂潜流带生境,为底栖微生物和大型底栖无脊椎动物提供了良好的栖息环境,并加快了生源物质的潜流交换速率,提高了水体的自净能力[74]。
4.2 水生动物主导的联合作用机制
水生动物对潜流带生境的影响与植物相比较小。水生动物通过某些物理或生物作用对水生生物群落和多样性产生影响,主要包括鱼类活动和无脊椎动物活动分别对其他生物生境的影响,以及不同种类动物间的捕食关系对潜流交换的影响。
生活在潜流带中的鱼类生命活动可以改变沉积物的物理和生化特性,为部分微生物提供合适的生境。研究表明,鱼类排泄物的输入能够提高底栖生物可摄取的营养物质含量,引起潜流带生物多样性的变化,例如,Pettigrew等[93]通过户外对比试验分析得出,鱼类粪便输入改变了河流底层藻类的初级生产,从而对上层甲壳类和沉积甲壳类的物种产生不同的影响。此外,鱼类可以通过产卵活动增强潜流带生态系统的生境异质性,从而使得生物多样性增强[78]。一些鱼类产卵场的存在会扰乱沉积物-水界面,并对潜流带中微生物和大型无脊椎动物数量和物种组成起到正面或负面作用[94-95]。鱼类作为许多淡水双壳贝类幼虫阶段特定的宿主,其存在还会限制贝类的多样性[96],这些鱼类的生命活动潜在地影响了部分生源物质的潜流交换通量。
潜流带中大型底栖无脊椎动物的取食、营巢等生命活动会改变沉积物中溶解氧和其他营养物质的分布[97],并刺激沉积物中好氧和厌氧微生物的生命活动进程[98-100]。大型无脊椎动物啃食生物膜、挖掘沉积物等行为,促进了区域优势流的形成,增加了底栖生物膜与水的接触,从而提高了生物膜的附着面积和菌群密度[39, 101],使得底栖微生物在无脊椎动物存在的情况下消耗了更多的溶解有机物和营养物[78]。
此外,水生动物间的捕食作用也会对潜流交换产生一定影响。一些肉食性鱼类和掠食性无脊椎动物以许多水生动物为食,从而对潜流带生物多样性产生了负面影响[78],进而作用于潜流交换过程。此外,捕食者的存在还可能改变潜流区域内被捕食者的行为,诱导生源物质从沉积物扩散到水中或周围的河岸带[102-104]。
5 总结与展望
底栖生物膜、水生动物和水生植物都可能直接或间接地影响潜流交换过程,近年来随着技术的发展,有关潜流带水力学和生态学交叉的研究越来越多。潜流交换和水生生物生态功能及群落多样性的关系逐渐被深入探索,并在一定程度上揭示了这种多样性模式背后的机制。尽管如此,该领域仍然存在众多问题有待进一步系统地研究。本文认为以下几个方面将是今后研究的重点:
(1) 潜流交换与水生生物互馈作用机制。潜流交换与水生生物生命活动之间的相互作用主要体现在2个方面: 一方面,潜流交换改变了生源物质的分布,影响了水生生境,如潜流交换对营养物质的储存和再释放能为鲑鱼提供稳定的营养源;另一方面,水生生物的活动可以直接作用于潜流交换通量,或者通过改变河床地形、渗透系数和孔隙度诱导泵吸交换,间接改变潜流交换。然而,潜流交换与水生生物互馈作用的研究尚停留于定性分析阶段,未来的研究宜考虑季节性水文变化、水利工程建设运行、特殊地形结构等条件,定量分析不同工况下潜流交换与水生生物互馈关系,以完善这方面的理论。
(2) 水生生态功能与地表-地下水相互作用关系。物质交换、能量循环等功能变化对外在生态环境的影响理论和定量化研究较为缺乏。例如,底栖微生物形成的生物膜是一个复杂的生物群落,在生态功能和生物多样性方面都有重要意义。生物膜的结构和功能与潜流交换所施加的物理作用密切相关,同时生物膜也能改变潜流交换的动力学特征。潜流交换对底栖生物膜传质功能有重要影响,甚至可能导致整个生态系统功能和生物-地球-化学过程改变。但由于水生生物与潜流交换关系的定量研究过于概化,生物群落发展对潜流交换作用的响应机制尚不明确。
(3) 河流潜流带的生物-地球-化学耦合过程的研究。潜流带是化学、生物物质频繁交换和生物化学作用强烈的区域,利用生物-地球-化学耦合的模式在研究物质循环、能量流动和信息传递等生态学内容方面有突出的优势。尽管现场试验和室内试验能提供较为可信的数据,试验仍可能存在诸多不确定因素。试验设计的改进是潜流带生物-地球-化学过程的一个研究方向。另外,利用数值模拟方法研究水力学作用和生态环境的关系也是一个重要手段,能有效地节约试验周期和试验资源,为未来河流生态规划提供参考依据。潜流交换的水力学与水生生物的生态环境作用耦合数值模拟方法研究,也将是未来的研究方向之一。
-
-
[1] RUTERE C, POSSELT M, HO A, et al. Biodegradation of metoprolol in oxic and anoxic hyporheic zone sediments: unexpected effects on microbial communities[J]. Applied Microbiology and Biotechnology, 2021, 105(14/15): 6103-6115.
[2] ALLEY W M, HEALY R W, LABAUGH J W, et al. Flow and storage in groundwater systems[J]. Science, 2002, 296(5575): 1985-1990. doi: 10.1126/science.1067123
[3] 袁兴中, 罗固源. 溪流生态系统潜流带生态学研究概述[J]. 生态学报, 2003, 23(5): 956-964. doi: 10.3321/j.issn:1000-0933.2003.05.017 YUAN X Z, LUO G Y. A brief review for ecological studies on hyporheic zone of stream ecosystem[J]. Acta Ecologica Sinica, 2003, 23(5): 956-964. (in Chinese) doi: 10.3321/j.issn:1000-0933.2003.05.017
[4] 金光球, 李凌. 河流中潜流交换研究进展[J]. 水科学进展, 2008, 19(2): 285-293. doi: 10.3321/j.issn:1001-6791.2008.02.021 JIN G Q, LI L. Advancement in the hyporheic exchange in rivers[J]. Advances in Water Science, 2008, 19(2): 285-293. (in Chinese) doi: 10.3321/j.issn:1001-6791.2008.02.021
[5] KO J, LEE J, KANG H. Effects of micro-topography on N2O emission from sediments in temperate streams[J]. Ecological Engineering, 2020, 155: 105906. doi: 10.1016/j.ecoleng.2020.105906
[6] TRUCHY A, SARREMEJANE R, MUOTKA T, et al. Habitat patchiness, ecological connectivity and the uneven recovery of boreal stream ecosystems from an experimental drought[J]. Global Change Biology, 2020, 26(6): 3455-3472. doi: 10.1111/gcb.15063
[7] SU X R, JIM YEH T C, SHU L C, et al. Scale issues and the effects of heterogeneity on the dune-induced hyporheic mixing[J]. Journal of Hydrology, 2020, 590: 125429. doi: 10.1016/j.jhydrol.2020.125429
[8] ZHOU T, ENDRENY T. The straightening of a river meander leads to extensive losses in flow complexity and ecosystem services[J]. Water, 2020, 12(6): 1680. doi: 10.3390/w12061680
[9] 金光球, 魏杰, 张向洋, 等. 平原河流水沙界面生源物质迁移转化过程及水环境调控的研究进展[J]. 水科学进展, 2019, 30(3): 434-444. doi: 10.14042/j.cnki.32.1309.2019.03.013 JIN G Q, WEI J, ZHANG X Y, et al. Advances in biogenic elements transport at the interface of stream-streambed and water environmental control in the plain river[J]. Advances in Water Science, 2019, 30(3): 434-444. (in Chinese) doi: 10.14042/j.cnki.32.1309.2019.03.013
[10] JIN G Q, TANG H W, GIBBES B, et al. Transport of nonsorbing solutes in a streambed with periodic bedforms[J]. Advances in Water Resources, 2010, 33(11): 1402-1416. doi: 10.1016/j.advwatres.2010.09.003
[11] JIN G Q, TANG H W, LI L, et al. Hyporheic flow under periodic bed forms influenced by low-density gradients[J]. Geophysical Research Letters, 2011, 38(22): L22401.
[12] JIN G Q, TANG H W, LI L, et al. Prolonged river water pollution due to variable-density flow and solute transport in the riverbed[J]. Water Resources Research, 2015, 51(4): 1898-1915. doi: 10.1002/2014WR016369
[13] JIN G Q, ZHANG Z T, LI R Z, et al. Transport of zinc ions in the hyporheic zone: experiments and simulations[J]. Advances in Water Resources, 2020, 146: 103775. doi: 10.1016/j.advwatres.2020.103775
[14] JIN G Q, JIANG Q H, TANG H W, et al. Density effects on nanoparticle transport in the hyporheic zone[J]. Advances in Water Resources, 2018, 121: 406-418. doi: 10.1016/j.advwatres.2018.09.004
[15] COOK S, PRICE O, KING A, et al. Bedform characteristics and biofilm community development interact to modify hyporheic exchange[J]. Science of the Total Environment, 2020, 749: 141397. doi: 10.1016/j.scitotenv.2020.141397
[16] 夏继红, 林俊强, 陈永明, 等. 国外河流潜流层研究的发展过程及研究方法[J]. 水利水电科技进展, 2013, 33(4): 73-77. https://www.cnki.com.cn/Article/CJFDTOTAL-SLSD201304023.htm XIA J H, LIN J Q, CHEN Y M, et al. Advancing processes and methods of abroad research on hyporheic zone[J]. Advances in Science and Technology of Water Resources, 2013, 33(4): 73-77. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-SLSD201304023.htm
[17] STANFORD J A, GAUFIN A R. Hyporheic communities of two montana rivers[J]. Science, 1974, 185(4152): 700-702. doi: 10.1126/science.185.4152.700
[18] MOLZ F, WIDDOWSON M, BENEFIELD L. Simulation of microbial growth dynamics coupled to nutrient and oxygen transport in porous media[J]. Water Resources Research, 1986, 22(8): 1207-1216. doi: 10.1029/WR022i008p01207
[19] BOULTON A J, FINDLAY S, MARMONIER P, et al. The functional significance of the hyporheic zone in streams and rivers[J]. Annual Review of Ecology and Systematics, 1998, 29: 59-81. doi: 10.1146/annurev.ecolsys.29.1.59
[20] AUBENEAU A F, HANRAHAN B, BOLSTER D, et al. Biofilm growth in gravel bed streams controls solute residence time distributions[J]. Journal of Geophysical Research: Biogeosciences, 2016, 121(7): 1840-1850. doi: 10.1002/2016JG003333
[21] SHRIVASTAVA S, STEWARDSON M J, ARORA M. Influence of bioturbation on hyporheic exchange in streams: conceptual model and insights from laboratory experiments[J]. Water Resources Research, 2021, 57(2): e2020WR028468.
[22] YUAN Y, CHEN X B, CARDENAS M B, et al. Hyporheic exchange driven by submerged rigid vegetation: a modeling study[J]. Water Resources Research, 2021, 57(6): e2019WR026675.
[23] STUBBINGTON R. The hyporheic zone as an invertebrate refuge: a review of variability in space, time, taxa and behaviour[J]. Marine and Freshwater Research, 2012, 63(4): 293-311. doi: 10.1071/MF11196
[24] WIMPENNY J, MANZ W, SZEWZYK U. Heterogeneity in biofilms[J]. FEMS Microbiology Reviews, 2000, 24(5): 661-671. doi: 10.1111/j.1574-6976.2000.tb00565.x
[25] GIFFORD S, DUNSTAN R H, O'CONNOR W, et al. Aquatic zooremediation: deploying animals to remediate contaminated aquatic environments[J]. Trends in Biotechnology, 2007, 25(2): 60-65. doi: 10.1016/j.tibtech.2006.12.002
[26] BATTIN T J, BESEMER K, BENGTSSON M M, et al. The ecology and biogeochemistry of stream biofilms[J]. Nature Reviews Microbiology, 2016, 14(4): 251-263. doi: 10.1038/nrmicro.2016.15
[27] GERBERSDORF S U, WIEPRECHT S. Biostabilization of cohesive sediments: revisiting the role of abiotic conditions, physiology and diversity of microbes, polymeric secretion, and biofilm architecture[J]. Geobiology, 2015, 13(1): 68-97. doi: 10.1111/gbi.12115
[28] PING X, JIN M G, XIAN Y. Effect of bioclogging on the nitrate source and sink function of a hyporheic zone[J]. Journal of Hydrology, 2020, 590: 125425. doi: 10.1016/j.jhydrol.2020.125425
[29] ROY CHOWDHURY S, ZARNETSKE J P, PHANIKUMAR M S, et al. Formation criteria for hyporheic anoxic microzones: assessing interactions of hydraulics, nutrients, and biofilms[J]. Water Resources Research, 2020, 56(3): e2019WR025971.
[30] BESEMER K. Biodiversity, community structure and function of biofilms in stream ecosystems[J]. Research in Microbiology, 2015, 166(10): 774-781. doi: 10.1016/j.resmic.2015.05.006
[31] PINTELON T R R, PICIOREANU C, LOOSDRECHT M C, et al. The effect of biofilm permeability on bio-clogging of porous media[J]. Biotechnology and Bioengineering, 2012, 109(4): 1031-1042. doi: 10.1002/bit.24381
[32] DRESCHER K, SHEN Y, BASSLER B L, et al. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(11): 4345-4350. doi: 10.1073/pnas.1300321110
[33] XIAN Y, JIN M G, ZHAN H B, et al. Reactive transport of nutrients and bioclogging during dynamic disconnection process of stream and groundwater[J]. Water Resources Research, 2019, 55(5): 3882-3903. doi: 10.1029/2019WR024826
[34] CARUSO A, BOANO F, RIDOLFI L, et al. Biofilm-induced bioclogging produces sharp interfaces in hyporheic flow, redox conditions, and microbial community structure[J]. Geophysical Research Letters, 2017, 44(10): 4917-4925. doi: 10.1002/2017GL073651
[35] SHOGREN A J, TANK J, HANRAHAN B, et al. Controls on fine particle retention in experimental streams[J]. Freshwater Science, 2020, 39: 28-38. doi: 10.1086/707396
[36] STEWART P S, FRANKLIN M J. Physiological heterogeneity in biofilms[J]. Nature Reviews Microbiology, 2008, 6(3): 199-210. doi: 10.1038/nrmicro1838
[37] BATTIN T J, KAPLAN L A, NEWBOLD J D, et al. Effects of current velocity on the nascent architecture of stream microbial biofilms[J]. Applied and Environmental Microbiology, 2003, 69(9): 5443-5452. doi: 10.1128/AEM.69.9.5443-5452.2003
[38] CURRAN J C, HESSION W C. Vegetative impacts on hydraulics and sediment processes across the fluvial system[J]. Journal of Hydrology, 2013, 505: 364-376. doi: 10.1016/j.jhydrol.2013.10.013
[39] BATTIN T J, KAPLAN L A, DENIS NEWBOLD J, et al. Contributions of microbial biofilms to ecosystem processes in stream mesocosms[J]. Nature, 2003, 426(6965): 439-442. doi: 10.1038/nature02152
[40] SÁNCHEZ-PÉREZ J M, MONTUELLE B, MOUCHET F, et al. Role of the hyporheic heterotrophic biofilm on transformation and toxicity of pesticides[J]. Annales De Limnologie-International Journal of Limnology, 2013, 49(2): 87-95. doi: 10.1051/limn/2013041
[41] SINGER G, BESEMER K, HOCHEDLINGER G, et al. Monomeric carbohydrate uptake and structure-function coupling in stream biofilms[J]. Aquatic Microbial Ecology, 2011, 62(1): 71-83. doi: 10.3354/ame01454
[42] 宁冰玉, 邹雯静, 赵闻卓, 等. 水生动物缺氧诱导因子研究进展[J]. 水产学杂志, 2022, 35(4): 110-116. doi: 10.3969/j.issn.1005-3832.2022.04.017 NING B Y, ZOU W J, ZHAO W Z, et al. A review: progress in research on hypoxia inducible factors in aquatic animals[J]. Chinese Journal of Fisheries, 2022, 35(4): 110-116. (in Chinese) doi: 10.3969/j.issn.1005-3832.2022.04.017
[43] ADÁMEK Z, MARŠÁLEK B. Bioturbation of sediments by benthic macroinvertebrates and fish and its implication for pond ecosystems: a review[J]. Aquaculture International, 2013, 21(1): 1-17. doi: 10.1007/s10499-012-9527-3
[44] BUXTON T H, BUFFINGTON J M, TONINA D, et al. Modeling the influence of salmon spawning on hyporheic exchange of marine-derived nutrients in gravel stream beds[J]. Canadian Journal of Fisheries and Aquatic Sciences, 2015, 72(8): 1146-1158. doi: 10.1139/cjfas-2014-0413
[45] PERNECKER B, MAUCHART P, CSABAI Z J E E. What to do if streams go dry?Behaviour of balkan goldenring (cordulegaster heros, odonata) larvae in a simulated drought experiment in sw hungary[J]. Ecological Entomology, 2020, 45(6): 1457-1465. doi: 10.1111/een.12931
[46] MARMONIER P, ARCHAMBAUD G, BELAIDI N, et al. The role of organisms in hyporheic processes: gaps in current knowledge, needs for future research and applications[J]. Annales De Limnologie-International Journal of Limnology, 2012, 48(3): 253-266. doi: 10.1051/limn/2012009
[47] 王璐, 杨海军, 李昆, 等. 长白山源头溪流底栖动物群落结构季节动态[J]. 生态学报, 2018, 38(13): 4834-4842. https://www.cnki.com.cn/Article/CJFDTOTAL-STXB201813028.htm WANG L, YANG H J, LI K, et al. Seasonal dynamics of macroinvertebrate community structure in a headwater stream in the Changbai Mountains[J]. Acta Ecologica Sinica, 2018, 38(13): 4834-4842. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-STXB201813028.htm
[48] GARRETT-WALKER J, COLLIER K J. Characterisation of constructed ponds and factors influencing their macroinvertebrate communities on the Lower Waikato River floodplain, New Zealand[J]. New Zealand Journal of Marine and Freshwater Research, 2021, 55(4): 550-564. doi: 10.1080/00288330.2020.1826987
[49] CARIOU M, FRANCOIS C M, VOISIN J, et al. Effects of bioturbation by tubificid worms on biogeochemical processes, bacterial community structure and diversity in heterotrophic wetland sediments[J]. Science of the Total Environment, 2021, 795: 148842. doi: 10.1016/j.scitotenv.2021.148842
[50] STUBBINGTON R, WOOD P J, REID I, et al. Benthic and hyporheic invertebrate community responses to seasonal flow recession in a groundwater-dominated stream[J]. Ecohydrology, 2011, 4(4): 500-511. doi: 10.1002/eco.168
[51] MACDONALD M J, ALBERTSON L K, POOLE G C. Ecosystem engineering in the streambed: net-spinning caddisflies influence hydraulic properties[J]. Ecohydrology, 2021, 14(2): e2266.
[52] MAO R C, WU J T, QIN X, et al. The effect of tubificid bioturbation on vertical water exchange across the sediment-water interface[J]. Water, 2020, 12(12): 3467. doi: 10.3390/w12123467
[53] NOGARO G, MERMILLOD-BLONDIN F, FRANCOIS-CARCAILLET F, et al. Invertebrate bioturbation can reduce the clogging of sediment: an experimental study using infiltration sediment columns[J]. Freshwater Biology, 2006, 51(8): 1458-1473. doi: 10.1111/j.1365-2427.2006.01577.x
[54] SAWYER A H, CARDENAS M B. Hyporheic flow and residence time distributions in heterogeneous cross-bedded sediment[J]. Water Resources Research, 2009, 45(8): w08406.
[55] CARDENAS M B, FORD A E, KAUFMAN M H, et al. Hyporheic flow and dissolved oxygen distribution in fish nests: the effects of open channel velocity, permeability patterns, and groundwater upwelling[J]. Journal of Geophysical Research: Biogeosciences, 2016, 121(12): 3113-3130. doi: 10.1002/2016JG003381
[56] BOANO F, HARVEY J W, MARION A, et al. Hyporheic flow and transport processes: mechanisms, models, and biogeochemical implications[J]. Reviews of Geophysics, 2014, 52(4): 603-679. doi: 10.1002/2012RG000417
[57] MONTGOMERY D, BUFFINGTON J, PETERSON N, et al. Stream-bed scour, egg burial depths, and the influence of salmonid spawning on bed surface mobility and embryo survival[J]. Canadian Journal of Fisheries and Aquatic Sciences, 1996, 53(5): 1061-1070. doi: 10.1139/f96-028
[58] TONINA D, BUFFINGTON J M. A three-dimensional model for analyzing the effects of salmon redds on hyporheic exchange and egg pocket habitat[J]. Canadian Journal of Fisheries and Aquatic Sciences, 2009, 66(12): 2157-2173. doi: 10.1139/F09-146
[59] PLEDGER A G, RICE S P, MILLETT J. Bed disturbance via foraging fish increases bedload transport during subsequent high flows and is controlled by fish size and species[J]. Geomorphology, 2016, 253: 83-93. doi: 10.1016/j.geomorph.2015.09.021
[60] PLEDGER A G, RICE S P, MILLETT J. Reduced bed material stability and increased bedload transport caused by foraging fish: a flume study with juvenile Barbel (Barbus barbus)[J]. Earth Surface Processes and Landforms, 2014, 39: 1500-1513.
[61] STATZNER B, SAGNES P. Crayfish and fish as bioturbators of streambed sediments: assessing joint effects of species with different mechanistic abilities[J]. Geomorphology, 2008, 93(3/4): 267-287.
[62] JONES K L, POOLE G C, WOESSNER W W, et al. Geomorphology, hydrology, and aquatic vegetation drive seasonal hyporheic flow patterns across a gravel-dominated floodplain[J]. Hydrological Processes, 2008, 22(13): 2105-2113. doi: 10.1002/hyp.6810
[63] MERRITT D M, SCOTT M L, LEROY POFF N, et al. Theory, methods and tools for determining environmental flows for riparian vegetation: riparian vegetation-flow response guilds[J]. Freshwater Biology, 2010, 55(1): 206-225. doi: 10.1111/j.1365-2427.2009.02206.x
[64] GHISALBERTI M. Mixing layers and coherent structures in vegetated aquatic flows[J]. Journal of Geophysical Research Atmospheres, 2002, 107(C2): 3011. doi: 10.1029/2001JC000871
[65] HEPPELL C M, WHARTON G, COTTON J A C, et al. Sediment storage in the shallow hyporheic of lowland vegetated river reaches[J]. Hydrological Processes, 2009, 23(15): 2239-2251. doi: 10.1002/hyp.7283
[66] BERGAMASCO A, de NAT L, FLINDT M R, et al. Interactions and feedbacks among phytobenthos, hydrodynamics, nutrient cycling and sediment transport in estuarine ecosystems[J]. Continental Shelf Research, 2003, 23(17/18/19): 1715-1741.
[67] DING M D, WAN J F, LI Q, et al. Literature review: how in-channel aquatic vegetation (IAV) affects hyporheic exchange (HE)[J]. IOP Conference Series: Materials Science and Engineering, 2020, 768(3): 032052. doi: 10.1088/1757-899X/768/3/032052
[68] WANG C, YU J Y, WANG P F, et al. Flow structure of partly vegetated open-channel flows with eelgrass[J]. Journal of Hydrodynamics (Ser B), 2009, 21(3): 301-307. doi: 10.1016/S1001-6058(08)60150-X
[69] SINISCALCHI F, NIKORA V I, ABERLE J. Plant patch hydrodynamics in streams: mean flow, turbulence, and drag forces[J]. Water Resources Research, 2012, 48(1): w01513.
[70] MCMILLAN S K, TUTTLE A K, JENNINGS G D, et al. Influence of restoration age and riparian vegetation on reach-scale nutrient retention in restored urban streams[J]. JAWRA Journal of the American Water Resources Association, 2014, 50(3): 626-638. doi: 10.1111/jawr.12205
[71] SALEHIN M, PACKMAN A I, WÖRMAN A. Comparison of transient storage in vegetated and unvegetated reaches of a small agricultural stream in Sweden: seasonal variation and anthropogenic manipulation[J]. Advances in Water Resources, 2003, 26(9): 951-964. doi: 10.1016/S0309-1708(03)00084-8
[72] CAMPOREALE C, PERUCCA E, RIDOLFI L, et al. Modeling the interactions between river morphodynamics and riparian vegetation[J]. Reviews of Geophysics, 2013, 51(3): 379-414. doi: 10.1002/rog.20014
[73] MALARD F, CAPDERREY C, CHURCHEWARD B, et al. Geomorphic influence on intraspecific genetic differentiation and diversity along hyporheic corridors[J]. Freshwater Biology, 2017, 62(12): 1955-1970. doi: 10.1111/fwb.13040
[74] 吕小央, 张松贺, 刘凯辉, 等. 水生植物-生物膜体系的生态功能与互作机制研究进展[J]. 水资源保护, 2015, 31(2): 20-25. https://www.cnki.com.cn/Article/CJFDTOTAL-SZYB201502004.htm LYU X Y, ZHANG S H, LIU K H, et al. Advances in ecological function and interaction mechanism of aquatic macrophyte-biofilm system[J]. Water Resources Protection, 2015, 31(2): 20-25. (in Chinese) https://www.cnki.com.cn/Article/CJFDTOTAL-SZYB201502004.htm
[75] TAI X N, MACKAY D S, SPERRY J S, et al. Distributed plant hydraulic and hydrological modeling to understand the susceptibility of riparian woodland trees to drought-induced mortality[J]. Water Resources Research, 2018, 54(7): 4901-4915. doi: 10.1029/2018WR022801
[76] MAGLIOZZI C, GRABOWSKI R, PACKMAN A, et al. Scaling down hyporheic exchange flows: from catchments to reaches[J]. Hydrology and Earth System Sciences Discussions, 2017: 1-53. doi: 10.5194/hess-2017-468
[77] WHITE D S, HENDRICKS S P. Lotic macrophytes and surface-subsurface exchange processes[M]//Streams and Ground Waters. Amsterdam: Elsevier, 2000: 363-379.
[78] PALMER M A, COVICH A P, LAKE S, et al. Linkages between aquatic sediment biota and life above sediments as potential drivers of biodiversity and ecological processes: a disruption or intensification of the direct and indirect chemical, physical, or biological interactions between aquatic sediment biota and biota living above the sediments may accelerate biodiversity loss and contribute to the degradation of aquatic and riparian habitats[J]. BioScience, 2000, 50(12): 1062-1075. doi: 10.1641/0006-3568(2000)050[1062:LBASBA]2.0.CO;2
[79] GREGORY S V, SWANSON F J, MCKEE W A, et al. An ecosystem perspective of riparian zones[J]. BioScience, 1991, 41(8): 540-551. doi: 10.2307/1311607
[80] HUSSNER A, STIERS I, VERHOFSTAD M J J M, et al. Management and control methods of invasive alien freshwater aquatic plants: a review[J]. Aquatic Botany, 2017, 136: 112-137. doi: 10.1016/j.aquabot.2016.08.002
[81] CARR J, D'ODORICO P, MCGLATHERY K, et al. Stability and bistability of seagrass ecosystems in shallow coastal lagoons: role of feedbacks with sediment resuspension and light attenuation[J]. Journal of Geophysical Research Atmospheres, 2010, 115(G3): G03011.
[82] GURNELL A. Plants as river system engineers[J]. Earth Surface Processes and Landforms, 2014, 39(1): 4-25. doi: 10.1002/esp.3397
[83] LAW R. A review of the function and uses of, and factors affecting, stream phytobenthos[J]. Freshwater Reviews, 2011, 4(2): 135-166. doi: 10.1608/FRJ-4.1.448
[84] VYMAZAL J, BGŘEZINOVÁ T D. Removal of nutrients, organics and suspended solids in vegetated agricultural drainage ditch[J]. Ecological Engineering, 2018, 118: 97-103. doi: 10.1016/j.ecoleng.2018.04.013
[85] MVLLER M, MENTEL M, van HELLEMOND J J, et al. Biochemistry and evolution of anaerobic energy metabolism in eukaryotes[J]. Microbiology and Molecular Biology Reviews: MMBR, 2012, 76(2): 444-495. doi: 10.1128/MMBR.05024-11
[86] ANDERSEN A N. Responses of ant communities to disturbance: five principles for understanding the disturbance dynamics of a globally dominant faunal group[J]. The Journal of Animal Ecology, 2019, 88(3): 350-362. doi: 10.1111/1365-2656.12907
[87] WILLIAMS D D. The ecology of temporary waters[M]. Berlin: Springer Science&Business Media, 2012.
[88] STRAYER D L. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future[J]. Freshwater Biology, 2010, 55: 152-174. doi: 10.1111/j.1365-2427.2009.02380.x
[89] WALKER P D, WIJNHOVEN S, van DER VELDE G. Macrophyte presence and growth form influence macroinvertebrate community structure[J]. Aquatic Botany, 2013, 104: 80-87. doi: 10.1016/j.aquabot.2012.09.003
[90] MENEZES S, BAIRD D J, SOARES A M V M. Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring[J]. Journal of Applied Ecology, 2010, 47(4): 711-719. doi: 10.1111/j.1365-2664.2010.01819.x
[91] STATZNER B, BÊCHE L A. Can biological invertebrate traits resolve effects of multiple stressors on running water ecosystems?[J]. Freshwater Biology, 2010, 55: 80-119. doi: 10.1111/j.1365-2427.2009.02369.x
[92] VANDEWALLE M, BELLO F, BERG M P, et al. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms[J]. Biodiversity and Conservation, 2010, 19(10): 2921-2947. doi: 10.1007/s10531-010-9798-9
[93] PETTIGREW C T, HANN B J, GOLDSBOROUGH L G. Waterfowl feces as a source of nutrients to a prairie wetland: responses of microinvertebrates to experimental additions[J]. Hydrobiologia, 1997, 362(1/2/3): 55-66.
[94] CUCHEROUSSET J, OLDEN J D. Ecological impacts of nonnative freshwater fishes[J]. Fisheries, 2011, 36(5): 215-230. doi: 10.1080/03632415.2011.574578
[95] HOLOMUZKI J R, FEMINELLA J W, POWER M E. Biotic interactions in freshwater benthic habitats[J]. Journal of the North American Benthological Society, 2010, 29(1): 220-244. doi: 10.1899/08-044.1
[96] HAAG W. North American freshwater mussels[M]. Cambridge: Cambridge University Press, 2012.
[97] MORAD M R, KHALILI A, ROSKOSCH A, et al. Quantification of pumping rate of Chironomus plumosus larvae in natural burrows[J]. Aquatic Ecology, 2010, 44(1): 143-153. doi: 10.1007/s10452-009-9259-2
[98] BERTICS V J, ZIEBIS W. Biodiversity of benthic microbial communities in bioturbated coastal sediments is controlled by geochemical microniches[J]. The ISME Journal, 2009, 3(11): 1269-1285. doi: 10.1038/ismej.2009.62
[99] HÖLKER F, VANNI M J, KUIPER J J, et al. Tube-dwelling invertebrates: tiny ecosystem engineers have large effects in lake ecosystems[J]. Ecological Monographs, 2015, 85(3): 333-351. doi: 10.1890/14-1160.1
[100] BARANOV V, LEWANDOWSKI J, ROMEIJN P, et al. Effects of bioirrigation of non-biting midges (Diptera: Chironomidae) on lake sediment respiration[J]. Scientific Reports, 2016, 6: 27329. doi: 10.1038/srep27329
[101] PERALTA-MARAVER I, REISS J, ROBERTSON A L. Interplay of hydrology, community ecology and pollutant attenuation in the hyporheic zone[J]. Science of the Total Environment, 2018, 610/611: 267-275. doi: 10.1016/j.scitotenv.2017.08.036
[102] LECERF A, RICHARDSON J. Assessing the functional importance of large-bodied invertebrates in experimental headwater streams[J]. Oikos, 2011, 120(6): 950-960. doi: 10.1111/j.1600-0706.2010.18942.x
[103] EFFENBERGER M, DIEHL S, GERTH M, et al. Patchy bed disturbance and fish predation independently influence the distribution of stream invertebrates and algae[J]. The Journal of Animal Ecology, 2011, 80(3): 603-614. doi: 10.1111/j.1365-2656.2011.01807.x
[104] SIH A, BOLNICK D I, LUTTBEG B, et al. Predator-prey naïveté, antipredator behavior, and the ecology of predator invasions[J]. Oikos, 2010, 119(4): 610-621. doi: 10.1111/j.1600-0706.2009.18039.x
-
期刊类型引用(5)
1. 窦传彬,夏继红,魏杰,祖加翼,王玥,陈青生,杨萌卓. 边滩对分层河岸带潜流层溶质迁移过程的影响. 水科学进展. 2024(06): 972-982 .  本站查看
本站查看
2. 窦传彬,夏继红,王玥,季书一. 河岸带潜流层空间结构对生源要素迁移转化的影响. 人民黄河. 2023(06): 79-85 .  百度学术
百度学术
3. 夏军强,朱恒,邓珊珊,周美蓉. 长江石首段河岸带地下水位变化过程模拟及分析. 水科学进展. 2023(04): 572-584 .  本站查看
本站查看
4. 段文生,俞彦,王玮,贾宇航,郭烨. 基于自然的河湖生态韧性修复探讨. 人民黄河. 2023(S2): 73-74+76 .  百度学术
百度学术
5. 王龙飞,王子怡,李轶. 基于连通性恢复的潜流带生态修复研究进展. 水科学进展. 2022(06): 1009-1020 .  本站查看
本站查看
其他类型引用(3)





 下载:
下载: